Research in the Howell group encompasses projects involving method development, total synthesis and the interface of chemistry and biology. The two main foci are the synthesis and exploitation of unusual strained heterocycles and the preparation of glycosphingolipids that activate natural killer T (NKT) cells.
Synthesis and Reactions of Novel Strained Heterocycles
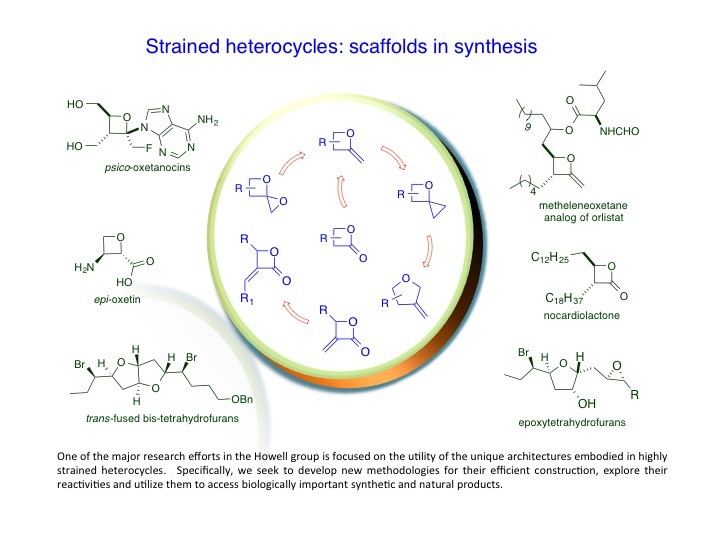
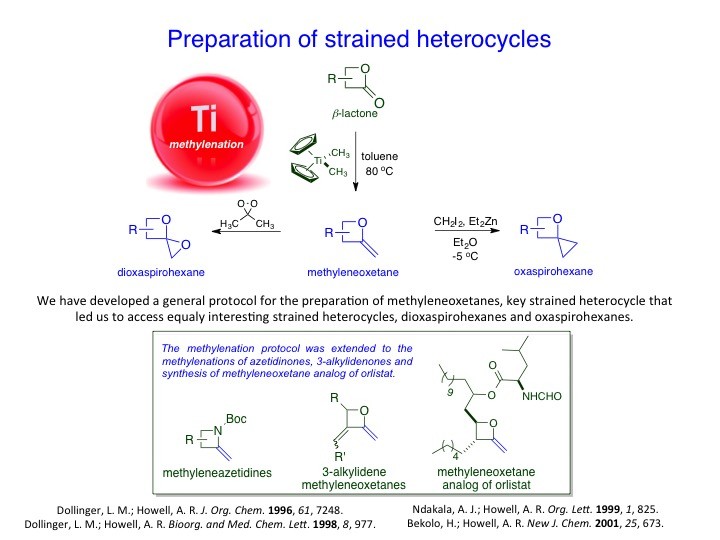
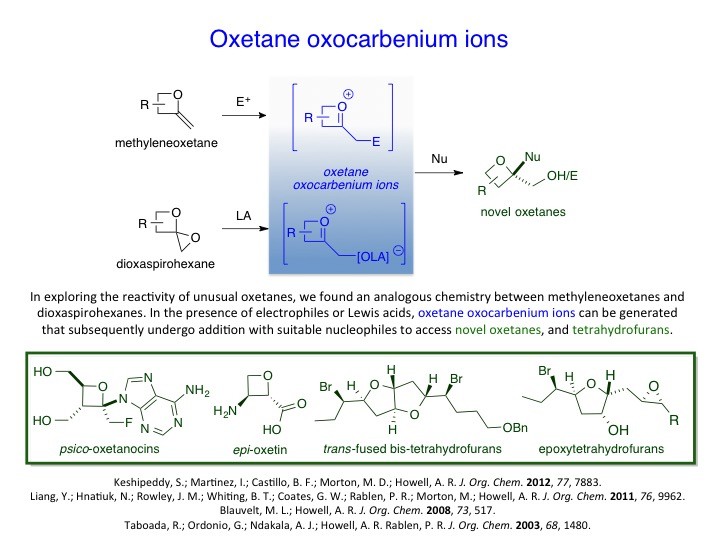
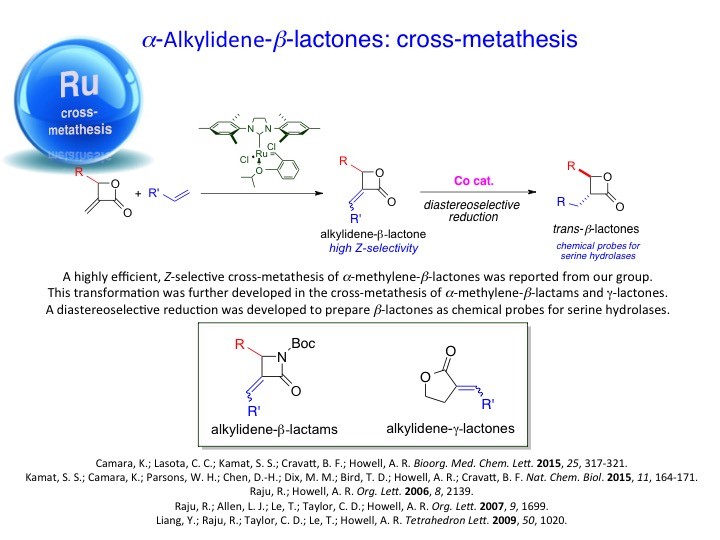
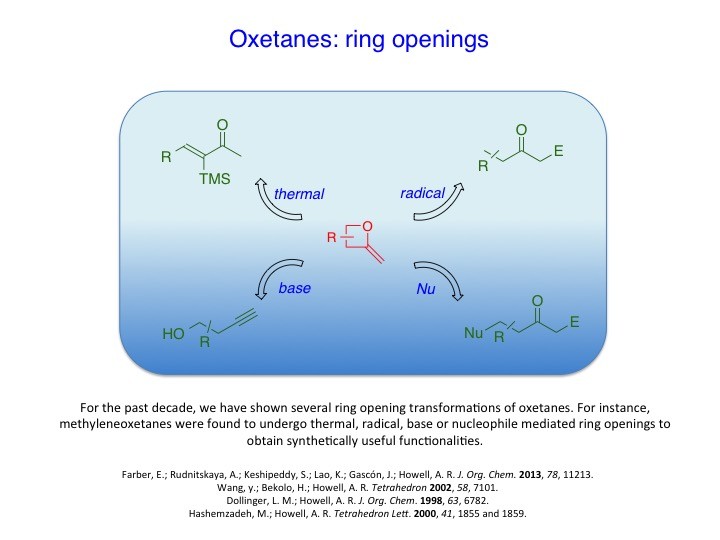
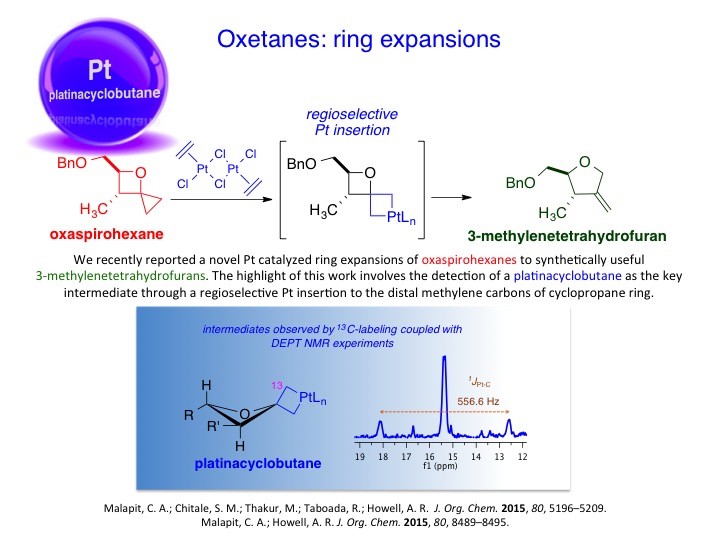
We have developed new methodologies to prepare unusual strained heterocycles in order to access biologically important natural products and their synthetic analogs and useful intermediates. For instance, α-methyleneoxetanes or dioxaspirohexanes have been utilized in the synthesis of homopropargylic alcohols, epi-oxetin, psico-oxetanocin analogs and laurenan-type polyethers. Likewise, the group has explored the cross-metathesis of α-methylene-β-lactones/lactams to access a variety of lactone/lactam containing compounds. These, too, have proved to be useful scaffolds for further studies. For example, α-methylene-β-lactones have been used as activity based probes for proteomic profiling of serine hydrolases.
Glycosphingolipids
Glycosphingolipids have been shown to modulate immune responses via activation of NKT cells, and this stimulation may have applications in the treatment of infectious diseases, cancer and autoimmune conditions. We have targeted natural and unnatural α-galactosyl ceramides and, more recently, natural and unnatural sulfatides as part of collaborations with immunologists to understand how these compounds can be used to elicit therapeutically relevant immune responses.